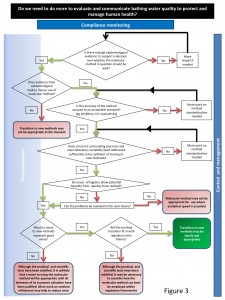
Decision-Making Framework
The overarching aim of the Working Group, through collaborative analysis of the current state of science and practice across these fields, was to integrate knowledge of cutting-edge innovative research in microbial quantification techniques with science-user needs. A significant element of this aim has been the development of a Decision-Making Framework (DMF) that provides a consistent, comprehensive and coordinated strategy for decision making. We define a DMF as a process for identifying and managing risks linked to an area of emerging concern, uncertainty or interest; in this case the subject being emerging molecular quantification tools for microbial parameters in EU regulated waters.
In constructing the DMF we have drawn on the expertise of the WG and a wide network of invited national and international experts whose microbiological knowledge and research activity complements key themes linked to:
- science and technology innovation;
- relevance to catchment management; and
- economic impacts on monitored waters, local economies, agencies and the state, all framed around commonly agreed science-user needs. The DMF will help users understand the current level of knowledge and ambiguity with regard to molecular quantification tools and how to formulate choice based on the current evidence-base.
Guidance on the use of the DMF
The DMF provides a coordinated, consistent and transparent approach to assist decision makers in weighing up the evidence for and against changing the method of analysis for bathing water compliance monitoring.
The DMF is hierarchical and iterativewith the starting point should be the epidemiological evidence base as this underpins the legitimacy of the method. The absence of such evidence immediately signals the need for further research before progress could be sanctioned. If the epidemiology is in place and it supports the adoption of a new method the next stage is to examine the accuracy of the method. Accreditation by an internationally recognised body and an ISO procedure would be evidence of standardisation that would support this criterion. Similarly there would be a need for precision within and between laboratories to be demonstrated on a routine basis. Even if the evidence base, accuracy and precision are robust, it may be that matters of logistics, i.e. sample transport time and laboratory space, negate the benefits offered by rapid methods. This will have to be weighed up and the likelihood of any problems being resolved in a useful timeframe will have to be explored. The cost effectiveness of method transition can then be asses as can the time frame within which it is achievable. Only if all of the above criteria are in place or can be established in a realistic time frame should the decision be made to move towards method transition. It is vital to understand that this DMF does not represent an instantaneous move from one analytical method to another. There will inevitable be a period during the transition when both culture and molecular methods will be used and the duration of this period will depend on many factors.